
Wara Samar
2nd June 2025
Darmiyan’s BrainSee Wins FDA Approval for Post-Market Surveillance Study to Advance Alzheimer’s Prognosis
More than seven million Americans live with Alzheimer’s disease today, a number projected to reach nearly 13 million by 2050, according to the Alzheimer’s Association. But the disease doesn’t impact patients alone. Families are stretched emotionally and financially. Caregivers often sacrifice their own health and careers. And healthcare systems face mounting costs as demand for long-term care continues to grow.
In the face of this escalating public health burden, the need for early, accurate, and accessible tools to assess Alzheimer’s risk has never been more urgent.
BrainSee: Predicting Alzheimer’s Progression Before It Happens
Darmiyan, a brain health technology company based in San Francisco, has developed BrainSee—an FDA-authorized, AI-powered clinical tool that helps physicians predict the likelihood of Alzheimer’s progression. Specifically, BrainSee forecasts whether patients over age 55 with amnestic mild cognitive impairment (aMCI) will develop clinical Alzheimer’s disease within five years.
Unlike conventional Alzheimer’s diagnostics that detect amyloid plaques, markers which don’t always correlate with disease progression, BrainSee is designed to differentiate between patients who are likely to remain stable and those who are not. Nearly 40% of amyloid-positive aMCI patients never develop Alzheimer’s, making early prognostic accuracy essential for avoiding unnecessary treatments and anxiety.
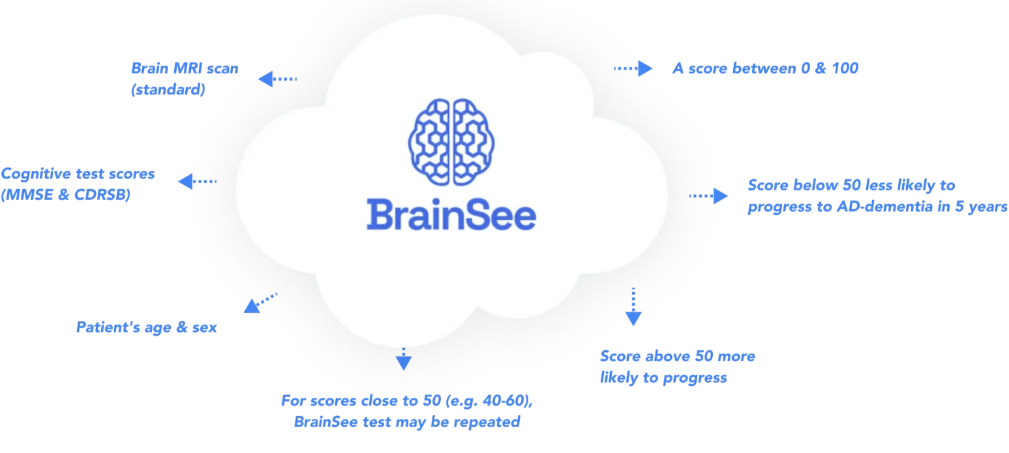
BrainSee integrates standard brain MRI, basic cognitive testing, age, and sex to generate a personalized prognostic score. Granted FDA marketing authorization in January 2024 through the De Novo pathway, it is currently the only non-invasive prognostic tool of its kind available for clinical use.
Crucially, the tool is designed for accessibility, allowing any licensed physician, including general practitioners, geriatricians, psychiatrists, and preventive medicine specialists, to order it. This positions BrainSee to expand cognitive risk assessment beyond specialty clinics and into everyday healthcare settings.
Darmiyan Launches Nationwide Study to Validate and Expand Alzheimer’s Prognostic Care
To build on BrainSee’s clinical promise, Darmiyan has now secured FDA approval for a 7-year post-market surveillance study. The study will focus on validating BrainSee’s safety, effectiveness, and real-world utility in a socio-demographically diverse patient population across the United States.
By partnering with leading institutions such as the National Alzheimer’s Coordinating Center (NACC), UC Davis and UAB Alzheimer’s Disease Research Centers, the Health & Aging Brain Study – Health Disparities (HABS-HD), the Alzheimer’s Disease Neuroimaging Initiative (ADNI), and Washington University’s OASIS dataset, Darmiyan aims to ensure representation from communities historically underrepresented in Alzheimer’s research.
The insights gained won’t only inform clinical practice; they will also help pharmaceutical companies and contract research organizations (CROs) identify the most suitable candidates for clinical trials. This could streamline the development of future Alzheimer’s treatments while promoting more equitable participation.
“With FDA approval of our post-market study, we aim to demonstrate BrainSee’s value in addressing one of the most pressing challenges in medicine: equitable access to high-quality, predictive care that guides dementia prevention, improves outcomes, and reduces costs,” said Dr. Kaveh Vejdani, Chief Medical and Technology Officer of Darmiyan in a statement. “We’re proud to work with these national leaders to ensure no community is left behind in the fight against Alzheimer’s disease.”
Toward a More Equitable Future in Alzheimer’s Diagnosis
The burden of Alzheimer’s is expected to grow dramatically in the coming decades. Technologies like BrainSee offer a new way forward—not just through earlier detection but by doing so in a way that includes more people, more providers, and more communities.
As Darmiyan continues its work, the coming years may offer clearer pathways for patients, families, and healthcare systems trying to navigate the uncertainties of cognitive decline.
Join Us at MedTech Malta 2025
At MedTech World, we’re excited to welcome you to MedTech Malta 2025, taking place from 12 to 14 November in the historic city of Valletta. This is our flagship event, where global leaders in healthcare innovation come together to share ideas, spark collaborations, and tackle some of the most pressing challenges in medicine today. We invite you to join us in Malta and be part of a community driving meaningful progress in MedTech. For more details, reach out to us at [email protected].





