Wara Samar
20th February 2025
Fixing Cartilage Damage Through Simple Surgery? Hy2Care’s Hydrogel Makes It Possible
For decades, cartilage damage has been a painful and often irreversible condition, leaving millions of patients with limited treatment options. Whether caused by sports injuries, aging, or degenerative diseases, the lack of effective regenerative solutions has caused many people to suffer from chronic osteoarthritis, resulting in many years of pain and disability. But what if there would be a way to effectively repair cartilage through a simple surgical procedure? There is with Hy2Care®, a Dutch MedTech company at the forefront of a groundbreaking approach. With its innovative hydrogel technology, Hy2Care is transforming the way cartilage injuries are treated, offering a minimally invasive surgical solution that could change the lives of patients worldwide.
At the heart of this innovation is Hy2Care’s CEO Leo Smit, whose vision and leadership are driving Hy2Care’s mission forward. As the company prepares to bring its technology to market, we spoke with Smit to explore the science behind Hy2Care®, the challenges of medical innovation, and what the future holds for cartilage repair.
Founded in 2014 as a spin-off of the Tech Med Centre at the University of Twente, Hy2Care® is pushing beyond the boundaries of regenerative medicine with its innovative hydrogel technology. At the centre of their research is the CartRevive® Hydrogel implant, an a-cellular resorbable implant designed to repair cartilage defects and restore joint function.

“Our hydrogel technology was developed by our co-founder Prof. Marcel Karperien and his team at the University of Twente,” explains Leo Smit. “The goal is to provide a natural scaffold for cells to integrate and rebuild tissue, addressing the limitations of existing treatments.”
With a Series A funding of €3.7 million in 2019, followed by additional investment and a Horizon 2020 EIC Accelerator grant Hy2Care® has expanded its research facilities and launched human clinical trials to validate its groundbreaking approach.
A Game-Changer in Cartilage Repair
The CartRevive® hydrogel implant is a novel approach to cartilage repair. Traditional treatments, such as microfracture surgery and debridement, often result in fibrous scar tissue rather than true cartilage. There are other effective treatments available, like Autologous Chondrocyte Implantation (ACI), a regenerative technology, however these are very expensive.
“Our hydrogel consists of natural polysaccharides with a composition mimicking that of substances present in the human joint, allowing for the formation of healthy, native cartilage without the need for additional restructuring of the implant. Unlike other scaffolding solutions, our implant degrades into nutrients for the body, allowing for effective and natural healing,” says Smit.
A key advantage of the CartRevive® hydrogel implant is its liquid state upon application. “The hydrogel conforms to the exact shape of the cartilage defect, eliminating the need for drilling or cutting,” Smit adds. “This ensures a perfect fit, good adhesion, and minimizes surgical complexity.”
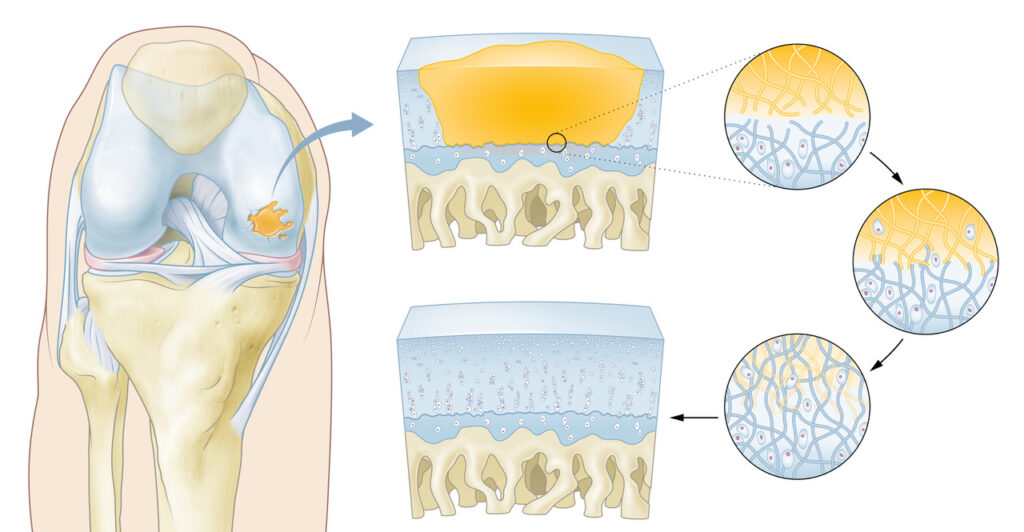
One of the most critical aspects of Hy2Care’s hydrogel is its enzymatic cross-linking process, which ensures complete biocompatibility. “The enzymatic cross-linking makes our solution 100% natural, eliminating concerns about foreign materials triggering immune responses,” Smit explains. “This is crucial for long-term integration and durability of the repaired tissue.”
Clinical Validation and Patient Outcomes
Hy2Care® has conducted extensive preclinical and clinical studies to validate the effectiveness of its implant. “We tested our concept in equine studies, as horse cartilage closely resembles human cartilage,” says Smit. “Within seven months, we observed nearly perfect cartilage restoration, comparable to best-in-class cell-based (regenerative) therapies but at a fraction of the cost.”
The results of the first-in-human trials have been equally promising. “MRI evaluations after 12 months show that defects treated with CartRevive® hydrogel implant are filled with smooth, natural cartilage tissue,” Smit reports. “Our KOOS (Knee Osteoarthritis Outcome Score), which measures pain reduction and mobility improvement, showed a 50% greater improvement than standard treatments.”
Target Patients and Future Applications
Currently, Hy2Care® is focusing on treating patients under 50 with traumatic cartilage injuries. “By intervening early, we can provide an effective repair fast and thus aim to prevent progression to osteoarthritis and the (future) need for joint replacement surgery,” Smit explains.
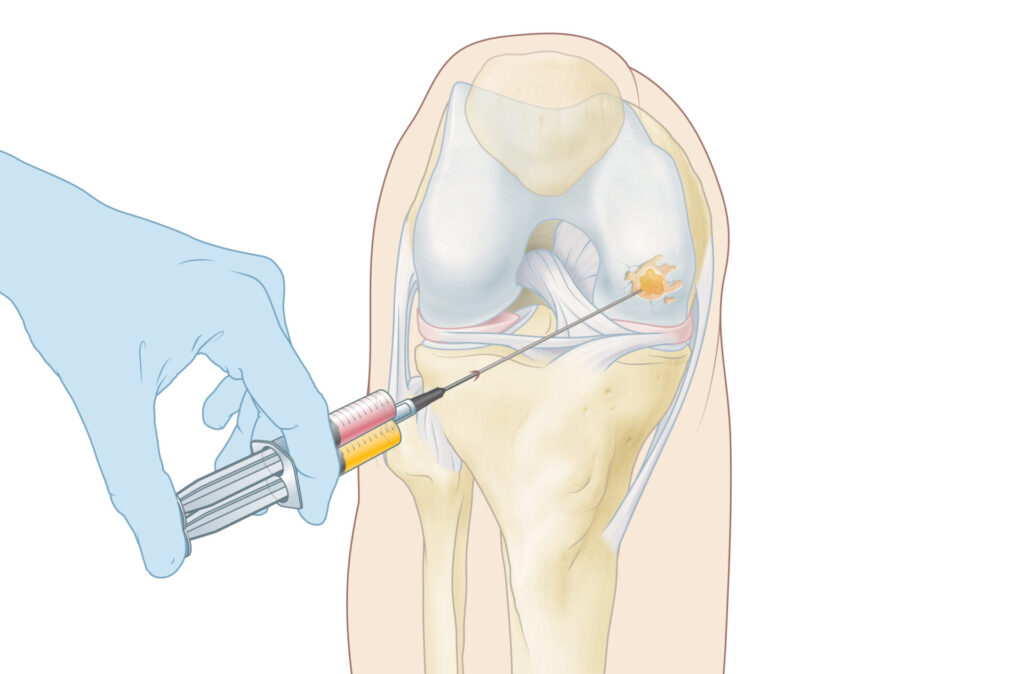
Looking ahead, Hy2Care® plans to expand its applications beyond knee cartilage repair. “We aim to address cartilage defects in the patella (knee-cap) as well as in other joints. There are also possibilities for applications outside orthopedics, which we hope to explore through partnerships,” Smit notes.
Hy2Care’s vision is to make effective cartilage repair widely accessible. For years, we have been led to believe that cartilage couldn’t heal. “But regenerative medicine has shown that natural healing is possible; it’s just been too expensive and complex for widespread use. Our CartRevive® hydrogel implant changes that,” says Smit.
Unlike traditional cell-based therapies, which require costly and time-consuming lab culturing, Hy2Care’s approach allows for in-situ restoration using the body’s own cells. “Our implant is an off-the-shelf solution that can be used in a single surgical procedure. This significantly lowers costs while providing high-quality repair outcomes,” Smit explains.
Commercialization and Funding Strategy
To bring the CartRevive® hydrogel implant to market, Hy2Care® is focused on securing regulatory approvals and scaling production. “We recently closed a convertible debt funding round and are now raising Series B funding,” Smit says. “Our goal is to initiate a U.S. clinical trial, launch in the EU, and cooperate with strategic partners to expand globally.”
The total funding target for Series B is approximately €35 million, with the first tranche expected to close late 2025. “We’re in discussions with – and also reaching out to – venture capital firms and strategic partners who can support with investment and our commercialization efforts,” Smit reveals.
Navigating Challenges in Healthcare Innovation
Despite the promising results, Smit acknowledges that commercialization comes with challenges. “Regulatory approval is just the first step,” he says. “Healthcare payers need to see cost-effectiveness before adopting new treatments.”
To address this, Hy2Care® is working closely with hospitals, insurers, and national health authorities to demonstrate the long-term benefits of its technology. “By preventing early-stage cartilage injuries from progressing to chronic osteoarthritis, we can reduce the need for costly joint replacements,” Smit explains.
“We believe in enabling the body to heal itself. By providing a simple, effective, and affordable solution, we are making regenerative healing accessible to more patients worldwide,” Smit concludes.