Michael Joe Cini
3rd July 2023
Pennsylvania Researchers Develop Magnetic Nanorobots for Selectively Eliminating Infective Fungal Species
A team of researchers at the University of Pennsylvania have recently developed nanorobots that can travel to the site of an infection from a fungal species, bind to the cells, and then release catalytic nanoparticles that destroy the fungus. The particles travel under the influence of an external magnetic field, which allows the team to direct them to specific locations in the body as determined by the team. So far, investigations have shown that the nanoparticles effectively destroy fungal aggregations, which are difficult to attack by conventional means.
Pennsylvania Develop Magnetic Nanorobots Target Fungal Treatment
Magnetic Nanorobots for Fungal Destruction by ROSs
In an innovative endeavour which involved the coupling of the latest nanozyme technology, antifungal therapy, and electromagnetism, an interdisciplinary team from the University of Pennsylvania’s dental and engineering departments have pioneered a new method for remedying fungal infections. Fungal infections are a significant public health risk, as recognised by the World Health Organization, with experts looking to prioritise them in approaches to improving health outcomes globally. Multiple solutions have been proposed as viable interventions for fungal infections, of which nanomaterials constitute a significant class.
Nanoparticles such as silver, gold, copper, sulfur, titanium dioxide, and zinc oxide have been tried to treat skin disorders such as candidiasis, pityriasis versicolor, and folliculitis, often with promising results. But while nanotech shows the possibility of being a valuable solution, many current iterations lack the potency and specificity needed for effective treatment. As a result, prolonged treatment times and off-target effects have sometimes occurred where such solutions were tried.
Now, this team is solving the problem of specificity and effective drug delivery with their solution. As put by Hyun (Michel) Koo, a Professor at Penn’s School of Dental Medicine and senior author of the paper, “Current antifungal therapies lack the potency and specificity required to quickly and effectively eliminate these pathogens, so this collaboration draws from our clinical knowledge and combines Ed’s team and their robotic expertise to offer a new approach.”
The creation of this solution required extensive collaboration between the medical and engineering teams, as acknowledged in Koo’s statement. The team members that developed the solution are part of Penn Dental’s Centre for Innovation and Precision Dentistry. This leading centre integrates engineering and computational approaches to discover new knowledge to mitigate diseases and develop new oral and craniofacial healthcare solutions. The centre is a hub for interdisciplinary collaboration between the University of Pennsylvania’s dental school and SEAS (School of Engineering and Applied Sciences), founded in 2021 as a collaborative effort between these departments.
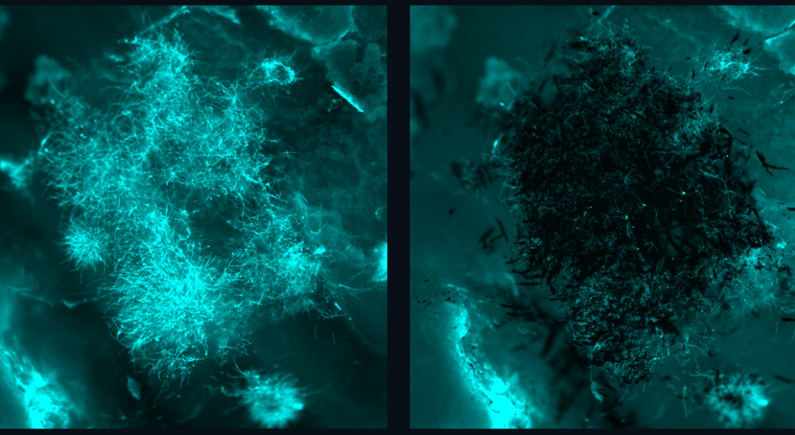
A significant element of the new technology was the way the team was able to solve the problem of specificity with the nanoparticles. “Our nanozyme assemblies show an incredible attraction to fungal cells, particularly when compared to human cells,” says Edward Steager, a researcher who worked on the project. The nanozymes can precisely accumulate where the fungal cells reside and provide rapid, targeted generation of ROSs (reactive oxygen species) at the site. Reactive oxygen species are highly cytotoxic and are able to rapidly eradicate the fungal cells in as little as ten minutes.
Microrobotics for Fungal Infections
The multi-disciplinary, multi-institutional team published their approaches and unprecedented findings in the May 2023 issue of the Wiley journal Advanced Materials. In the article, which is titled ‘Nanozyme-based robotics approach for targeting fungal infection,’ the researchers outline how they utilised an innovative approach of electromagnetic field frequency modulation and fine-scale spatiotemporal control to direct iron oxide nanozyme assemblies to sites with Candida albicans infection. They were able to harness the tunable properties of the nanozymes, together with selective binding, to carry out localised antifungal activity in animal tissue models.
To create the magnetic nanozyme assemblies, the team created a custom electromagnetic array with multi-axis motion, composed of two programmable electromagnets on either side of a container and an iron core at the centre of the electromagnet. The nanoparticle dispersions were then transferred to the container in the working space between the electromagnets, and a direct current was passed through the electromagnet to initiate the nanozyme-microrobot configurations. A magnetic field was generated within the container, and a programmable microcontroller manoeuvred the shape and motion of the microrobots.
The team also measured the catalytic activity of the microrobots, with peroxidase as a reference. Peroxidase is a biological enzyme that is produced by peroxisomes, which are subcellular structures dedicated to detoxification. The enzyme generates Oxygen from peroxide catalysis, which can form reactive oxygen species damaging to fungal cells. Reactive oxygen species can peroxidate lipids and cause oxidative DNA damage, leading to cell death.
When tested against Candida albicans, the most common pathogenic fungus in humans, ROS generation for five minutes effectively killed the fungus – more than ten times more effective than hydrogen peroxide. The nanozymes completely eradicated the fungi within ten minutes, dropping quantities of pathogenic cells to undetectable levels. The nanoparticles also showed little affinity for human cells and significant affinity for fungal biofilms, highlighting their specificity and safety for clinical use.
CiPD: A Notable Collaboration for Fungal Eradication
With contributions from researchers at two major departments at the University of Pennsylvania, the discovery was a collaborative effort from 11 researchers, with Edward Steager and Hyun Koo serving as corresponding authors for the project. Edward Steager is a senior research investigator at the University of Pennsylvania, having held a postdoc position at the University for four years, where he researched biological microrobotics at the GRASP lab. Hyun (Michel) Koo is the Director of the Centre for Innovation and Precision Dentistry, with a research focus on understanding how biofilms cause oral infectious diseases. He is also a professor in the Department of Orthodontics, working in the Pediatric Dentistry and Community Oral Health divisions.
Other research team members are Min Jun Oh, Seokyoung Yoon, Alaa Babeer, Yuan Liu, Zhi Ren, Zhenting Xiang, Yilan Miao, David Cormode, and Chider Chen. Min Jun Oh, Zhi Ren, Zhenting Xiang, and Yilan Miao are researchers at the School of Dental Medicine. On the other hand, David Cormode and Edward Steager are primarily affiliated with the School of Engineering and Applied Sciences at Penn. The National Institute for Dental and Craniofacial Research partly funded the research, with additional support from the Basic Science Research Program through the National Research Foundation of Korea’s Ministry of Education.
Med-Tech World Summit in Malta: Join Us
Be sure to mark your calendars for the upcoming Med-Tech World Summit on October 19th and 20th at the Mediterranean Conference Centre, Malta. This highly anticipated summit will offer a platform for further exploration and discussion of cutting-edge advancements in the field of medical technology, fostering collaboration and shaping the future of healthcare.




