Michael Joe Cini
24th November 2023
Medtronic is on a Roll! Two Devices Awarded Heartfelt Approval in Europe
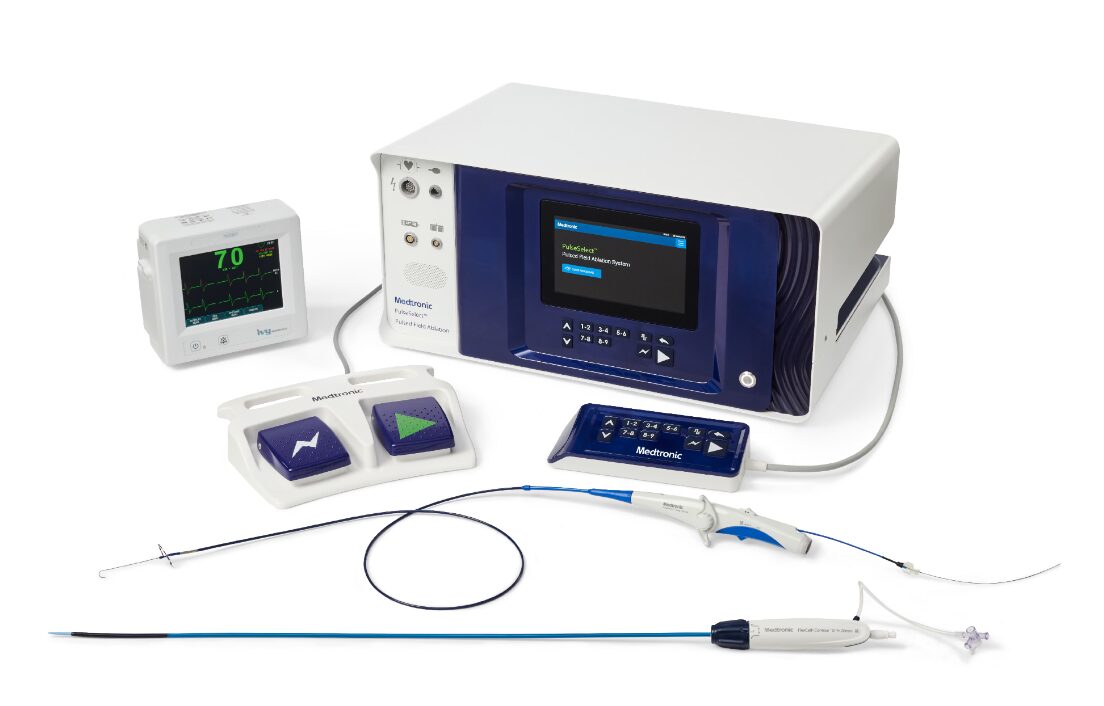
Medtronic, a world leader in health technology, has obtained European approval through the Conformité Européenne (CE) mark for its innovative devices that can treat a common heart condition called atrial fibrillation. The company announced that its PulseSelect and Nitron console devices will be launched in some European markets in early 2024.
The heart of the matter: Medtronic tech approved in Europe
The PulseSelect device has shown promising results in the prestigious clinical trial PULSED AF, where it achieved the desired outcome in just 30 seconds of energy delivery on average.
The device has received European approval based on the results of the PULSED AF study, which met its safety and effectiveness goals. The study involved two types of patients, those with occasional and those with persistent atrial fibrillation.
The data was presented at a major cardiology conference (ACC.23/WCC) and published in a prestigious journal, Circulation.
The Nitron console device is another device that has received the CE mark. It is a device that supports the existing range of Medtronic’s cardiac cryoablation catheters, which use cold energy to freeze the tissue that causes atrial fibrillation.
“With multiple CE Mark milestones, today’s announcement demonstrates our commitment to innovation and building a strong electrophysiology portfolio.” – Rebecca Seidel, president of the Cardiac Ablation Solutions business, which is part of the Cardiovascular Portfolio.
PulseSelect
PulseSelect is a revolutionary device transforming the landscape of atrial fibrillation treatment. Unlike other methods that involve potential risks through heat or cold, PulseSelect utilises pulses of electric fields to precisely target veins causing irregular heartbeats, ensuring minimal impact on surrounding heart structures.
What sets PulseSelect apart is its distinctive design, validated by the globally renowned PULSED AF study—one of the safest studies on heart ablation. PulseSelect achieves vein isolation with an impressive average total energy delivery time of just 30 seconds.
Beyond its effectiveness, PulseSelect is designed with user-friendliness in mind. It seamlessly integrates with any mapping system, or can even be used without one, simplifying the treatment process.
Renowned cardiologist and Professor of Cardiology, Dr. Lucas V.A. Boersma, highlights PulseSelect as a game-changer in AFib ablation. He applauds its safety measures and manoeuvrability, underscoring its unprecedented control over energy applications.
“The electrophysiology community is eagerly awaiting new innovation to enhance the safety and efficiency of AFib ablation,” says Dr. Boersma.
PulseSelect has surpassed expectations with an impressive safety record in its global pivotal study. The combination of innovation and safety sets a noteworthy standard in heart ablation, making it stand out in the field.
Nitron Console
The next-generation Nitron console has similarly earned the CE mark and is now supporting the commercially available Arctic Front™ and Freezor™ family of Cardiac Cryoablation Catheters.
This console introduces a range of innovative features, including, real-time procedural data capture, optimised workflow and an enhanced Graphical User Interface (GUI).
Khaldoun Tarakji, MD MPH, Chief Medical Officer of the Cardiac Ablation Solutions business at Medtronic, emphasised the commitment to patient care with this advanced technology, stating that “Every patient deserves the best care. What motivates all of us at Medtronic is the privilege of serving patients by empowering electrophysiologists globally with the safest and most effective ablation technologies.”
Innovation and market milestones
In 2022, the global electrophysiology ablation market reached a valuation of $2.7 billion, as reported by a market model from GlobalData. Projections indicate substantial growth, with expectations for the market to soar to $4.2 billion by 2033. Notably, cryoablation catheters are anticipated to contribute significantly, constituting a segment valued at $738 million.
Medtronic, holding a robust 17% global share in the electrophysiology ablation catheter market, achieved a pivotal milestone in March 2023 when it received European approval for its Affera mapping and ablation system.
Highlighting these achievements, Seidel stated, “These milestones are part of our investment in the future of our cryoablation franchise with Nitron, as well as the future of pulsed-field ablation with PulseSelect, following our more than ten years of scientific research and development.”
Anticipating a strategic move, both PulseSelect and Nitron are slated to be commercially available in select European countries early next calendar year, signifying a significant leap in cardiovascular technology.
November has proven to be a remarkable month for Medtronic in the cardiovascular sector. Notably, the company secured clearance from the US Food and Drug Administration (FDA) for its renal denervation system, solidifying its position alongside ReCor Medical as one of the only two companies with approved devices in the US for this highly anticipated therapy.
Med-Tech around the World!
Prepare for an exciting global roadshow series with Med-Tech World in 2024! The journey begins with a spectacular event in Dubai on February 26th, featuring cutting-edge content, gripping start-up pitches, and on-stage discussions with prominent investors. Throughout the year, the Med-Tech World Summit will then venture into new horizons, debuting events in Barcelona and Berlin.




